
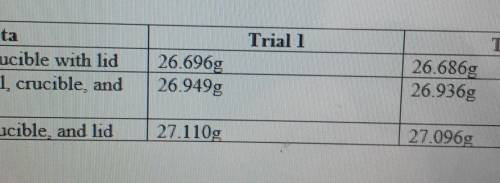
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this information, what is the theoretical yield of MgO (in both trials) with Magnesium as the limiting reactant.
What is the percent yield of MgO for each trial.
What is the average percent yield of MgO for both trials.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this...
Questions

Mathematics, 18.06.2020 09:57

Mathematics, 18.06.2020 09:57

Biology, 18.06.2020 09:57

Mathematics, 18.06.2020 09:57

English, 18.06.2020 09:57

Mathematics, 18.06.2020 09:57





Mathematics, 18.06.2020 09:57



Mathematics, 18.06.2020 09:57

History, 18.06.2020 09:57

History, 18.06.2020 09:57

Biology, 18.06.2020 09:57


English, 18.06.2020 09:57

Mathematics, 18.06.2020 09:57



