
Chemistry, 27.12.2020 14:00 aaroneduke4576
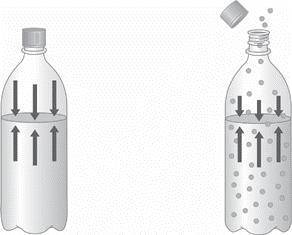
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
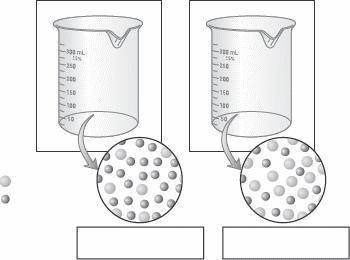
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or l...
Questions

English, 22.06.2021 19:00





Mathematics, 22.06.2021 19:00

English, 22.06.2021 19:00






Mathematics, 22.06.2021 19:00

Advanced Placement (AP), 22.06.2021 19:00

Mathematics, 22.06.2021 19:00

Biology, 22.06.2021 19:00


Mathematics, 22.06.2021 19:00


Mathematics, 22.06.2021 19:00



