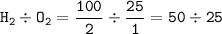Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
100cm^3 of hydrogen is mixed with 25cm^3 of oxygen at a temperature of 150*C. The gases react as sho...
Questions


Mathematics, 21.10.2020 23:01

English, 21.10.2020 23:01

Biology, 21.10.2020 23:01


Mathematics, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01

Social Studies, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01



Geography, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01




Mathematics, 21.10.2020 23:01


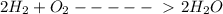 (All are gases)
(All are gases)