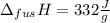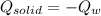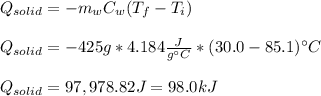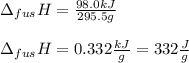Chemistry, 25.12.2020 19:20 didraga777
you take 295.5 g of a solid at 30.0 c and let it melt in 425 g of water. the water temperature decreases from 85.1 c to 30.0 c. calculate the heat of fusion of this solid

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
you take 295.5 g of a solid at 30.0 c and let it melt in 425 g of water. the water temperature decre...
Questions














English, 16.12.2019 17:31




Mathematics, 16.12.2019 17:31

English, 16.12.2019 17:31

Mathematics, 16.12.2019 17:31