Review Toples
References
Use the References to access important value in needed for its quest...

Chemistry, 25.12.2020 01:00 alazayjaime1423
Review Toples
References
Use the References to access important value in needed for its question
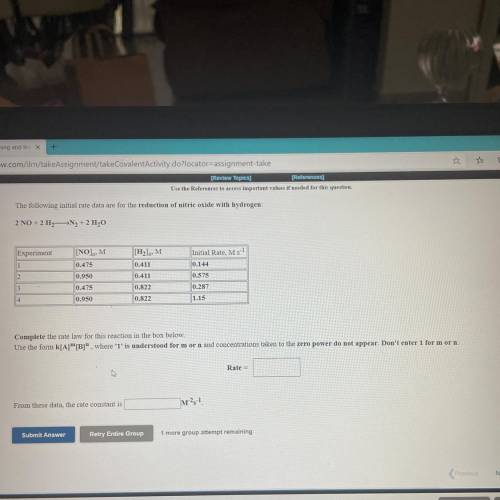
The following initial rate data are for the reduction of nitric oxide with hydrogen
2 NO 211N, 211,0
Experiment
1
INO).M
0,475
IM
0.411
mit Rate, M.
0.144
0.575
0.000
0.478
0.000
0,822
0.822
0.217
1.IS
1
Complete the rate law for this reaction in the box below
Use the form KIA"", where 'T' is understood for morn and concentrations taken to the zero power do not appear. Don't enter for morn
Rate
From these data, the rate constant is
m?!
Submit Answer
Retry Entre Group
1 more group attempt remaining

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 3

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Questions




Computers and Technology, 19.11.2021 22:50

Computers and Technology, 19.11.2021 22:50


Mathematics, 19.11.2021 22:50


Mathematics, 19.11.2021 22:50





History, 19.11.2021 22:50

Social Studies, 19.11.2021 22:50

Business, 19.11.2021 22:50

Mathematics, 19.11.2021 22:50


Mathematics, 19.11.2021 22:50

Mathematics, 19.11.2021 22:50



