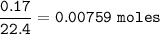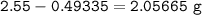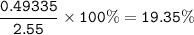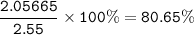Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
2.55 gram of an alloy of silver and zinc on treatment with excess dilute hydrochloric acid gave 170...
Questions

History, 04.02.2020 00:55


Physics, 04.02.2020 00:55



Biology, 04.02.2020 00:55

Mathematics, 04.02.2020 00:55

Mathematics, 04.02.2020 00:55


Mathematics, 04.02.2020 00:55



Mathematics, 04.02.2020 00:55



Mathematics, 04.02.2020 00:55

Mathematics, 04.02.2020 00:55

Health, 04.02.2020 00:55