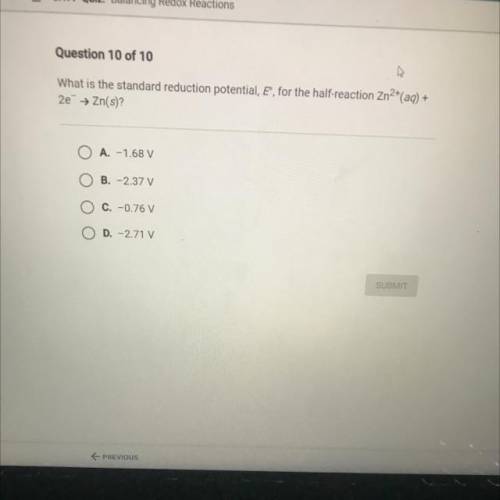
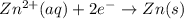What is the standard reduction potential, E, for the half-reaction Zn2+(aq)+2e- -> Zn(s)?
...

Chemistry, 24.12.2020 02:10 ciaralamont2
What is the standard reduction potential, E, for the half-reaction Zn2+(aq)+2e- -> Zn(s)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Questions





English, 24.02.2021 22:00

Mathematics, 24.02.2021 22:00


Mathematics, 24.02.2021 22:00


Social Studies, 24.02.2021 22:00

Mathematics, 24.02.2021 22:00


Biology, 24.02.2021 22:00


Business, 24.02.2021 22:00


Mathematics, 24.02.2021 22:00

Mathematics, 24.02.2021 22:00