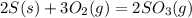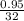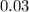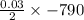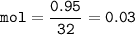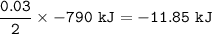Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
The value of ΔH° for the reaction below is -790 kJ. The enthalpy change accompanying the reaction of...
Questions

Computers and Technology, 13.07.2019 05:10


Computers and Technology, 13.07.2019 05:10



Computers and Technology, 13.07.2019 05:10








Medicine, 13.07.2019 05:10


Mathematics, 13.07.2019 05:10



Mathematics, 13.07.2019 05:10