
Chemistry, 23.12.2020 14:00 jacobdobson5856
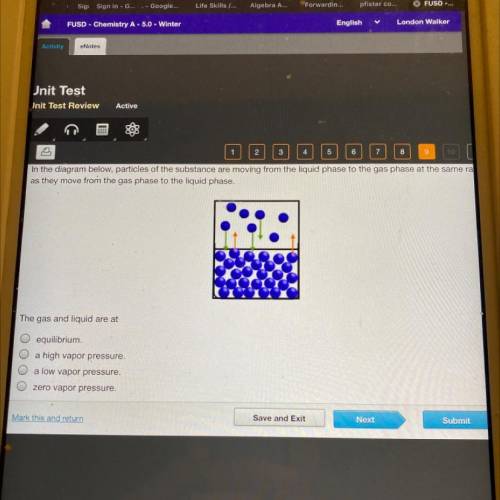
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase at the same ra
as they move from the gas phase to the liquid phase.
The gas and liquid are at
O equilibrium.
O a high vapor pressure.
O a low vapor pressure.
O zero vapor pressure.
Mark

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

You know the right answer?
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase a...
Questions








Mathematics, 15.11.2019 15:31




Mathematics, 15.11.2019 15:31

Mathematics, 15.11.2019 15:31


Mathematics, 15.11.2019 15:31



Computers and Technology, 15.11.2019 15:31

History, 15.11.2019 15:31



