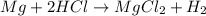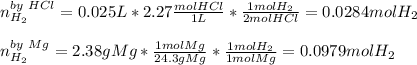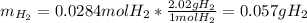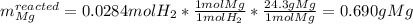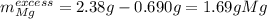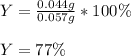1) When 2.38g of magnesium is added to 25.0cm of 2.27 M hydrochloric acid, hydrogen gas is released.
a) Determine the limiting reactant
b) Calculate the mass of hydrogen gas produced.
c) Calculate the mass of excess reactant remained at the end of reaction.
d) What is the percentage yield if 0.044g of hydrogen gas is obtained from the experiment?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

You know the right answer?
1) When 2.38g of magnesium is added to 25.0cm of 2.27 M hydrochloric acid, hydrogen gas is released....
Questions


Mathematics, 04.11.2020 04:30

Mathematics, 04.11.2020 04:30

History, 04.11.2020 04:30




Geography, 04.11.2020 04:30

Mathematics, 04.11.2020 04:30



Biology, 04.11.2020 04:30

Geography, 04.11.2020 04:30

Mathematics, 04.11.2020 04:30


Chemistry, 04.11.2020 04:30

Social Studies, 04.11.2020 04:30

Chemistry, 04.11.2020 04:30

History, 04.11.2020 04:30

SAT, 04.11.2020 04:30