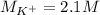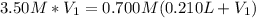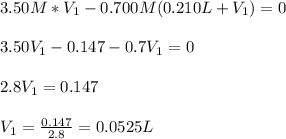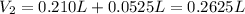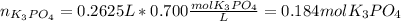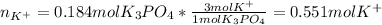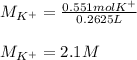Chemistry, 23.12.2020 04:30 neekobecky599
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water
t...
Questions

Geography, 23.06.2020 16:01

English, 23.06.2020 16:01




History, 23.06.2020 16:01

Mathematics, 23.06.2020 16:01

History, 23.06.2020 16:01

Biology, 23.06.2020 16:01



Mathematics, 23.06.2020 16:01

Computers and Technology, 23.06.2020 16:01


Mathematics, 23.06.2020 16:01



Mathematics, 23.06.2020 16:01