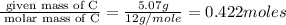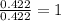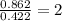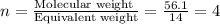Chemistry, 23.12.2020 01:00 kcameronanderso
When 6.040 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 18.95 grams of CO2 and 7.759 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 56.11 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

You know the right answer?
When 6.040 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 18.95 grams...
Questions



Mathematics, 26.05.2021 17:50

English, 26.05.2021 17:50


English, 26.05.2021 17:50




English, 26.05.2021 17:50


Social Studies, 26.05.2021 17:50


Health, 26.05.2021 17:50



Mathematics, 26.05.2021 17:50



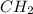 and molecular formula is
and molecular formula is 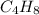
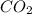 = 18.95 g
= 18.95 g
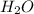 = 7.759 g
= 7.759 g
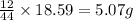 of carbon will be contained.
of carbon will be contained.
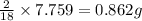 of hydrogen will be contained.
of hydrogen will be contained.