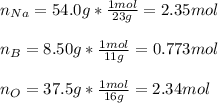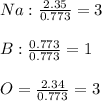Chemistry, 21.12.2020 18:10 JEThompson6416
What is the empirical formula for a compound that is comprised of 54.0% sodium, 8.50% boron, and 37.5% oxygen by mass?
A) Na2B2O3.
B) Na3BO3.
C) Na3B2O2.
D) Na4BO4.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1


Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
What is the empirical formula for a compound that is comprised of 54.0% sodium, 8.50% boron, and 37....
Questions

Mathematics, 14.07.2019 04:00


Biology, 14.07.2019 04:00


Mathematics, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00




Health, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00


Health, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00


Chemistry, 14.07.2019 04:00

Business, 14.07.2019 04:00

Mathematics, 14.07.2019 04:00