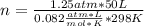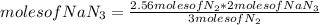Chemistry, 21.12.2020 17:40 deandrebryant89
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas from sodium azide. 2NaN3(s) -->2Na(s) + 3N2 (g)How many moles of sodium azide are needed to produce sufficient nitrogen to fill a 50.0 L air bag to a pressure of 1.25 atm at 25C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1
You know the right answer?
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas fr...
Questions






Advanced Placement (AP), 25.05.2020 00:00

Mathematics, 25.05.2020 00:00

English, 25.05.2020 00:00


English, 25.05.2020 00:00



Mathematics, 25.05.2020 00:00

History, 25.05.2020 00:00

Engineering, 25.05.2020 00:00



History, 25.05.2020 00:00

Mathematics, 25.05.2020 00:00

Mathematics, 25.05.2020 00:00

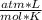 T= 25 C= 298 K
T= 25 C= 298 K