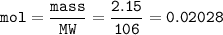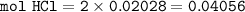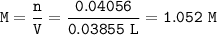Chemistry, 21.12.2020 16:00 ameliaxbowen7
If 38.55 mL of HCl is required to titrate 2.150 g of Na2CO3 according to the following equation, what is the molarity of the HCl solution? Na2CO3 + 2HCl (aq)→ 2NaCl (aq) + CO2 (g) + H20 (1)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
If 38.55 mL of HCl is required to titrate 2.150 g of Na2CO3 according to the following equation, wha...
Questions

Mathematics, 26.03.2020 19:06

History, 26.03.2020 19:06

Mathematics, 26.03.2020 19:06

Mathematics, 26.03.2020 19:06



Mathematics, 26.03.2020 19:06

History, 26.03.2020 19:06



Social Studies, 26.03.2020 19:06

Biology, 26.03.2020 19:06

Mathematics, 26.03.2020 19:06

History, 26.03.2020 19:06

Mathematics, 26.03.2020 19:06


History, 26.03.2020 19:06

Mathematics, 26.03.2020 19:06