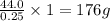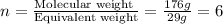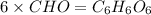Chemistry, 19.12.2020 16:10 amandaestevez030
Burning 12.00 g of an oxoacid produces 17.95 g of carbon dioxide and 4.87 g of water. Consider that 0.25 moles of oxoacid equals 44.0 g. For this compound, determine the empirical and molecular formula.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1
You know the right answer?
Burning 12.00 g of an oxoacid produces 17.95 g of carbon dioxide and 4.87 g of water. Consider that...
Questions

History, 19.05.2021 18:30




Mathematics, 19.05.2021 18:30

Social Studies, 19.05.2021 18:30


Mathematics, 19.05.2021 18:30

Social Studies, 19.05.2021 18:30

Mathematics, 19.05.2021 18:30

Mathematics, 19.05.2021 18:30




Mathematics, 19.05.2021 18:30

Biology, 19.05.2021 18:30




History, 19.05.2021 18:30

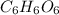
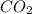 = 17.95 g
= 17.95 g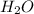 = 4.87 g
= 4.87 g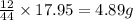 of carbon will be contained.
of carbon will be contained.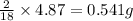 of hydrogen will be contained.
of hydrogen will be contained.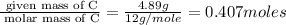
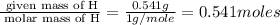
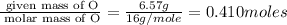
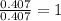
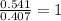
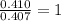
 .
.