
Chemistry, 19.12.2020 01:00 nshadow2920
If the reaction below produces 6 moles of H20, how many moles ofNH3 were required?2CH4 + 2NH3 + 302 --> 2HCN + 6H20 m | Enter your answer as a number only; no units.
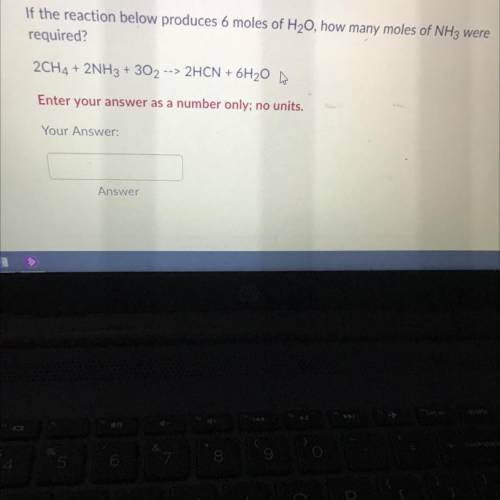

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1


Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
If the reaction below produces 6 moles of H20, how many moles ofNH3 were required?2CH4 + 2NH3 + 302...
Questions

Mathematics, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30

Biology, 19.04.2021 19:30

History, 19.04.2021 19:30


Mathematics, 19.04.2021 19:30

Social Studies, 19.04.2021 19:30


Mathematics, 19.04.2021 19:30


Mathematics, 19.04.2021 19:30





Mathematics, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30



History, 19.04.2021 19:30



