Why are metals good conductors of heat?
O A. The free-moving electrons transmit heat quickly.
...

Chemistry, 18.12.2020 05:50 kerarucker12pe384k
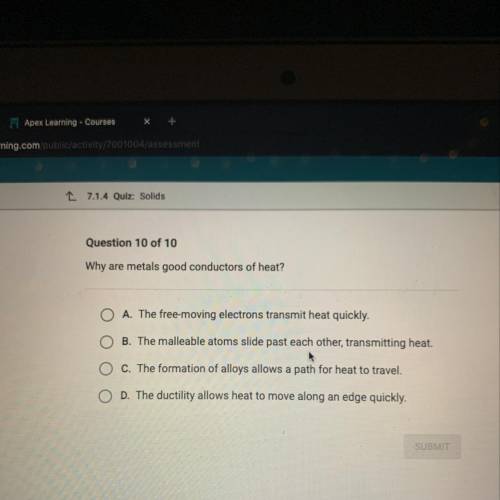
Why are metals good conductors of heat?
O A. The free-moving electrons transmit heat quickly.
B. The malleable atoms slide past each other, transmitting heat.
O c. The formation of alloys allows a path for heat to travel.
O D. The ductility allows heat to move along an edge quickly.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Questions

Mathematics, 06.10.2019 05:00


Biology, 06.10.2019 05:00

Chemistry, 06.10.2019 05:00


Mathematics, 06.10.2019 05:00

Biology, 06.10.2019 05:00



Social Studies, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00

Geography, 06.10.2019 05:00

Social Studies, 06.10.2019 05:00



Biology, 06.10.2019 05:00


Geography, 06.10.2019 05:00

Social Studies, 06.10.2019 05:00



