GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Ques...

Chemistry, 18.12.2020 04:00 sabahtramirez01
GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Question :
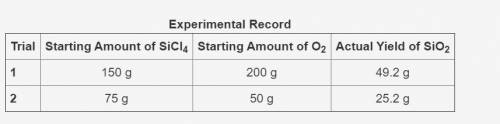
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Questions

History, 17.10.2020 14:01



English, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Social Studies, 17.10.2020 14:01



Geography, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

History, 17.10.2020 14:01

Social Studies, 17.10.2020 14:01

French, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Chemistry, 17.10.2020 14:01

Geography, 17.10.2020 14:01


English, 17.10.2020 14:01


Chemistry, 17.10.2020 14:01



