REALLY DONT BE THAT GUY

Chemistry, 18.12.2020 03:50 Makoshark6887
GIVING BRAIBLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
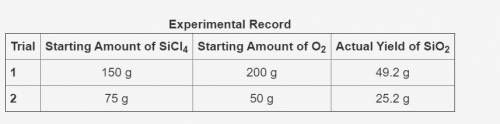
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
GIVING BRAIBLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
REALLY DONT BE THAT GUY
Questions

Mathematics, 28.01.2020 21:54

Mathematics, 28.01.2020 21:54


Computers and Technology, 28.01.2020 21:54

History, 28.01.2020 21:54

Social Studies, 28.01.2020 21:54


Computers and Technology, 28.01.2020 21:54

Mathematics, 28.01.2020 21:54



Spanish, 28.01.2020 21:54



Mathematics, 28.01.2020 21:54


Mathematics, 28.01.2020 21:54


Biology, 28.01.2020 21:54



