
Chemistry, 18.12.2020 01:00 reneebrown017
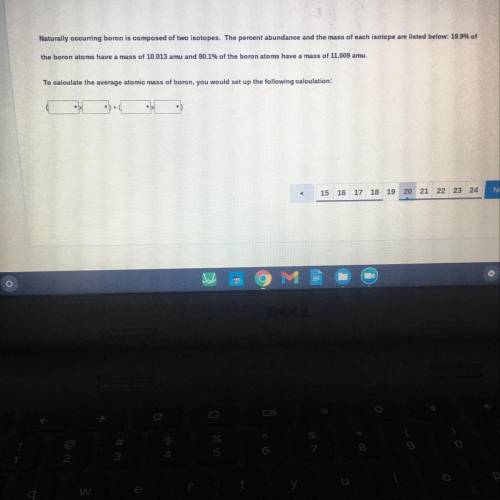
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each isotope are listed below: 19.9% of
ne boron atoms have a mass of 10.013 amu and 80.1% of the boron atoms have a mass of 11.009 amu.
calculate the average atomic mass of boron, you would set up the following calculation:
What

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each is...
Questions

Mathematics, 13.04.2020 22:54

Mathematics, 13.04.2020 22:54

Social Studies, 13.04.2020 22:54




History, 13.04.2020 22:54



Mathematics, 13.04.2020 22:54


Biology, 13.04.2020 22:54

Biology, 13.04.2020 22:54

Social Studies, 13.04.2020 22:54

History, 13.04.2020 22:54

Mathematics, 13.04.2020 22:54


Mathematics, 13.04.2020 22:54

History, 13.04.2020 22:54

History, 13.04.2020 22:54



