
Chemistry, 17.12.2020 20:00 mikurrjurdan
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and delta H rxn given above, which of the following can best be used to justify that the reaction is thermodynamically favorable at 298 K and constant pressure?
N2 (g) + 3H2 (g) --> 2NH3 (g)
K= 5.6 × 10^5 at 298 K
delta H rxn= -91.8 kj/mol
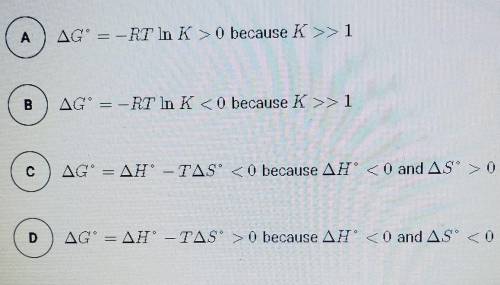

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and...
Questions


History, 27.01.2020 05:31


Biology, 27.01.2020 05:31


Mathematics, 27.01.2020 05:31

Mathematics, 27.01.2020 05:31


Biology, 27.01.2020 05:31


Mathematics, 27.01.2020 05:31



Social Studies, 27.01.2020 05:31

Mathematics, 27.01.2020 05:31




Mathematics, 27.01.2020 05:31



