
Chemistry, 17.12.2020 19:00 erinolson07cats
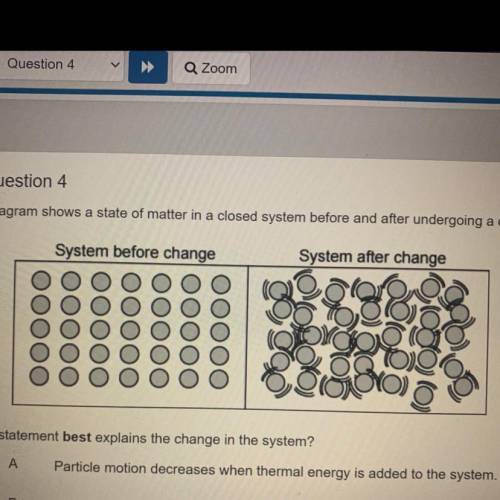
The diagram shows a state of matter in a closed system before and after undergoing a change.
System before change
System after change
Svo
Which statement best explains the change in the system?
Particle motion decreases when thermal energy is added to the system.
A
B
Particle motion increases when solid particles are added to the system.
С
Particle motion increases when thermal energy is added to the system.
D
Particle motion decreases when gas particles are added to the system.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
The diagram shows a state of matter in a closed system before and after undergoing a change.
System...
Questions

Mathematics, 14.12.2021 23:00

Mathematics, 14.12.2021 23:00

Mathematics, 14.12.2021 23:00



Biology, 14.12.2021 23:00



Mathematics, 14.12.2021 23:00

Geography, 14.12.2021 23:00

Mathematics, 14.12.2021 23:00


Mathematics, 14.12.2021 23:00

Mathematics, 14.12.2021 23:00

Social Studies, 14.12.2021 23:00




English, 14.12.2021 23:00



