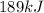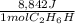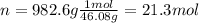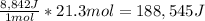Chemistry, 17.12.2020 09:50 debramknoxx
In an industrial process ethanol C2H60 burns with O2 to produce heat. Each mole of ethanol produces 8842 joules during the reaction.
C2H5OH (1) + 3 O2(g) 2 CO2(g) + 3 H2O(0) + 8842 Joules
How many Kilojoules are obtained from burning 982.6 g of ethanol?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1
You know the right answer?
In an industrial process ethanol C2H60 burns with O2 to produce heat. Each mole of ethanol produces...
Questions

Mathematics, 22.09.2019 01:50

Biology, 22.09.2019 01:50




English, 22.09.2019 01:50

Health, 22.09.2019 01:50


Mathematics, 22.09.2019 01:50

Biology, 22.09.2019 01:50

Spanish, 22.09.2019 01:50

Mathematics, 22.09.2019 01:50



Biology, 22.09.2019 01:50


Social Studies, 22.09.2019 01:50


Social Studies, 22.09.2019 01:50

Biology, 22.09.2019 01:50