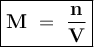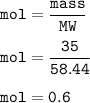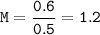Chemistry, 17.12.2020 03:10 babygirl10302015
A solution was prepared by dissolving 35.0 g of NaCl in water to make a 0.5 L solution. What is the molarity of the solution? The molar mass of NaCl = 58.44 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
A solution was prepared by dissolving 35.0 g of NaCl in water to make a 0.5 L solution. What is the...
Questions


Mathematics, 20.05.2021 19:50


Mathematics, 20.05.2021 19:50

Computers and Technology, 20.05.2021 19:50



Mathematics, 20.05.2021 19:50


Biology, 20.05.2021 19:50


Mathematics, 20.05.2021 19:50

Computers and Technology, 20.05.2021 19:50

Spanish, 20.05.2021 19:50


Mathematics, 20.05.2021 19:50



Mathematics, 20.05.2021 19:50

Mathematics, 20.05.2021 19:50