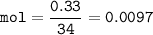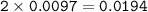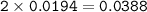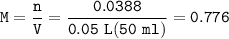Chemistry, 17.12.2020 02:30 getzperez1962
0.33 g of hydrosulfuric acid, H25, is added to 50.0 mL of water. What is the concentration (molarity) of [H301 of this solution?
H25 --> 2H +52
2H+ + 2 H20 --> 2 H30* + 2 OH
[Do not include units in your answer]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
0.33 g of hydrosulfuric acid, H25, is added to 50.0 mL of water. What is the concentration (molarity...
Questions




Biology, 28.09.2019 09:00

History, 28.09.2019 09:00


Health, 28.09.2019 09:00




Mathematics, 28.09.2019 09:00



Health, 28.09.2019 09:00




Mathematics, 28.09.2019 09:00

Chemistry, 28.09.2019 09:00