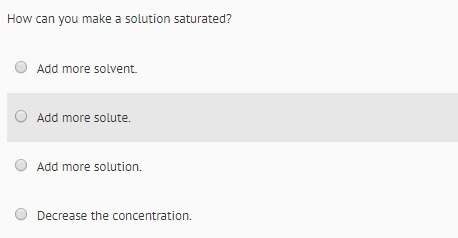
Chemistry, 17.12.2020 01:00 KayleighMorganhopkin
A compound is 26.06 % N and73.94 % O. If the compound's molar mass is 108.0 g/mol, what is its molecular formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
You know the right answer?
A compound is 26.06 % N and73.94 % O. If the compound's molar mass is 108.0 g/mol, what is its molec...
Questions


Mathematics, 26.10.2020 18:00

English, 26.10.2020 18:00

Mathematics, 26.10.2020 18:00


Social Studies, 26.10.2020 18:00


Social Studies, 26.10.2020 18:00

Mathematics, 26.10.2020 18:00

Health, 26.10.2020 18:00

English, 26.10.2020 18:00