Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
...

Chemistry, 16.12.2020 23:00 JoshuaXYP9978
Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
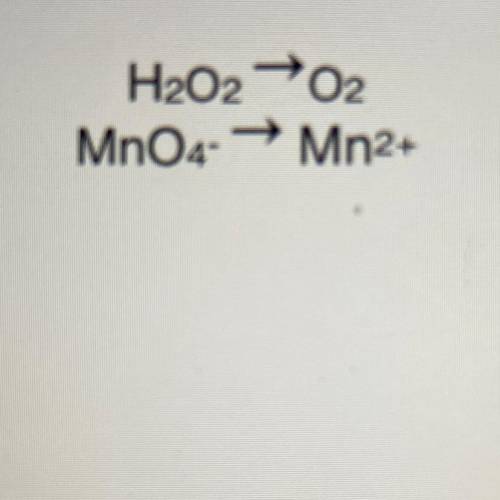

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

You know the right answer?
Questions





Mathematics, 29.04.2021 21:10




Mathematics, 29.04.2021 21:10



History, 29.04.2021 21:10

Mathematics, 29.04.2021 21:10




History, 29.04.2021 21:10





