Moles CO
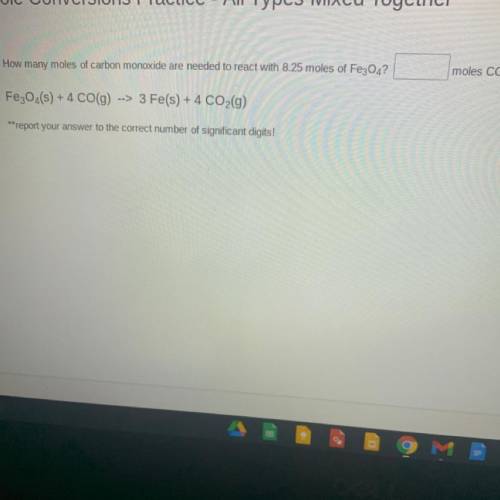
How many moles of carbon monoxide are needed to react with 8.25 moles of Fe3O4?
Fe3O...

Chemistry, 16.12.2020 19:30 hamilclips1748
Moles CO
How many moles of carbon monoxide are needed to react with 8.25 moles of Fe3O4?
Fe3O4(s) + 4 CO(9)
--> 3 Fe(s) + 4 CO2(g)
**report your answer to the correct number of significant digits!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3
You know the right answer?
Questions

Business, 18.02.2022 15:20



English, 18.02.2022 15:20


History, 18.02.2022 15:20

Biology, 18.02.2022 15:20

Biology, 18.02.2022 15:20





Chemistry, 18.02.2022 15:20


Business, 18.02.2022 15:30




Mathematics, 18.02.2022 15:30



