
Chemistry, 16.12.2020 16:40 5921000521
The mineral manganosite, manganese(ll) oxide, crystallizes in the rock salt structure the face-centered structure adopted by NaCl) with a density of 5.365 g/cm'. Find the unit cell edge length of manganosite.
A. 444.5 pm
B. 352.8 pm
C. 280.0 pm
D. 368.2 pm
E. 417.9 pm

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3
You know the right answer?
The mineral manganosite, manganese(ll) oxide, crystallizes in the rock salt structure the face-cente...
Questions


Mathematics, 24.05.2021 23:30


Mathematics, 24.05.2021 23:30

Mathematics, 24.05.2021 23:30


English, 24.05.2021 23:30


English, 24.05.2021 23:30








Arts, 24.05.2021 23:30



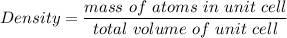
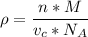
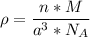
![[Mn(11)O] = 70.93 \ g/mol](/tpl/images/0990/1650/36da1.png)
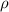 is given as 5.365 g/cm³
is given as 5.365 g/cm³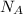 = 6.023 × 10²³ atoms/mol
= 6.023 × 10²³ atoms/mol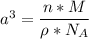
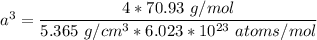
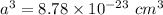
![a= \sqrt[3]{8.78 \times 10^{-23} \ cm^3}](/tpl/images/0990/1650/8dd66.png)


