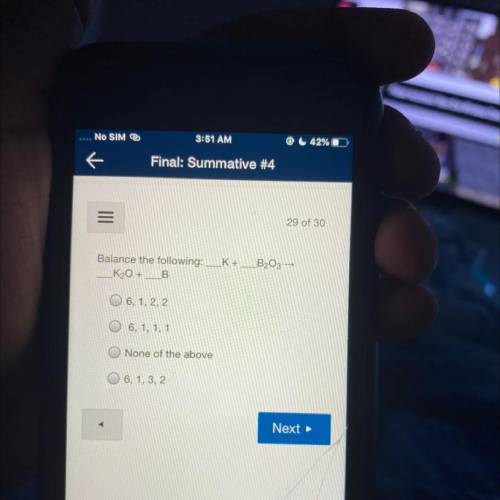
B203 >
Balance the following: K+
K2O +B
6, 1, 2, 2
6, 1, 1, 1
None of th...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

You know the right answer?
Questions



Mathematics, 20.10.2019 18:10

Mathematics, 20.10.2019 18:10


World Languages, 20.10.2019 18:10


Mathematics, 20.10.2019 18:10


Biology, 20.10.2019 18:10



History, 20.10.2019 18:10


History, 20.10.2019 18:10


Computers and Technology, 20.10.2019 18:10


SAT, 20.10.2019 18:10