
Chemistry, 16.12.2020 14:00 lesliealaves16
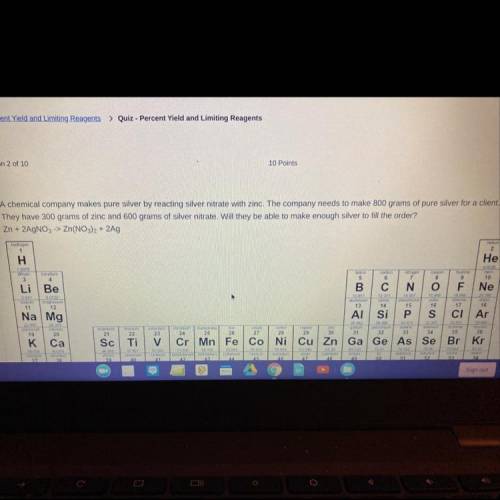
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make s00 grams of pure silver for a client.
They have 300 grams of zinc and 600 grams of silver nitrate. Will they be able to make enough silver to fil the order?
Zn + 2AgNO,> Zn(NO), + 2Ag A. Yes, there will be extra left over. B. Yes, they will have exactly enough C. No, the silver nitrate will run out first. D. No, the zinc nitrate will run out first

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

You know the right answer?
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make...
Questions


Biology, 07.07.2019 20:10




Mathematics, 07.07.2019 20:10

Chemistry, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

Biology, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

English, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10

Advanced Placement (AP), 07.07.2019 20:10


Mathematics, 07.07.2019 20:10

Mathematics, 07.07.2019 20:10




