
Chemistry, 16.12.2020 14:00 ashvinmsingh
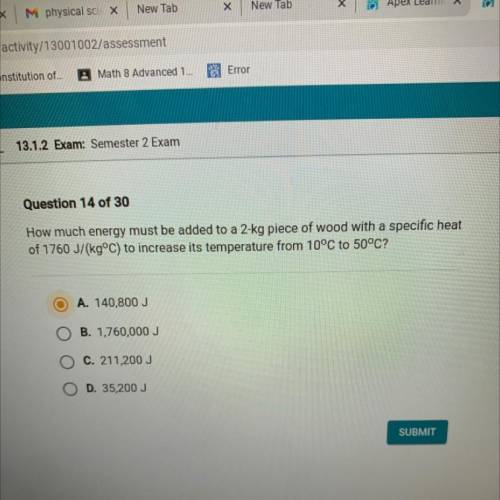
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to increase its temperature from 10°C to 50°C?
A. 140,800 J
B. 1,760,000 J
C. 211,200 J
D. 35,200 J

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to incr...
Questions

Mathematics, 30.04.2021 04:00


Mathematics, 30.04.2021 04:00

English, 30.04.2021 04:00


Mathematics, 30.04.2021 04:00

Mathematics, 30.04.2021 04:00




Mathematics, 30.04.2021 04:00





Mathematics, 30.04.2021 04:00

Mathematics, 30.04.2021 04:00

Chemistry, 30.04.2021 04:00




