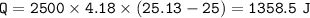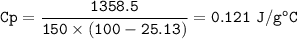Chemistry, 16.12.2020 08:10 kierafisher05
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 100 oC. After placing the weapon in the water, the temperature dropped to 25.13 oC. Find the specific heat capacity of the unknown metal by first calculating the heat energy gained by the water.
Water
Mass = 2500 g
Initial temperature = 25 o C Final temperature = 25.13 oC
Equations
q = m C p Δ T C p = q / (mΔT)
1. How much heat energy (q) did the water gain?
2. What is the specific heat capacity (C p) of the unknown metal?
3. Compare the heat capacity you calculated with the one below, which one matches?
Specific heat capacity of metal (J / g or C)
Silver 0.235
Gold 0.129 Lead 0.121
Copper
4. What metal was the murder weapon made of?
0.385

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3
You know the right answer?
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 10...
Questions


Biology, 28.01.2020 04:31



Mathematics, 28.01.2020 04:31




Mathematics, 28.01.2020 04:31