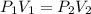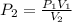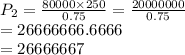The pressure inside of a sealed syringe of hydrogen gas is 80.0KPa with a volume of 250 Liters. What is the pressure inside
of the syringe after the plunger is pulled back far enough to make final volume of the syringe 0.750 Liters? (assume the
temperature and amount of gas in the container are held constant)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
The pressure inside of a sealed syringe of hydrogen gas is 80.0KPa with a volume of 250 Liters. What...
Questions

Spanish, 03.02.2021 01:00

Physics, 03.02.2021 01:00

English, 03.02.2021 01:00


Biology, 03.02.2021 01:00



Chemistry, 03.02.2021 01:00


Biology, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00



Advanced Placement (AP), 03.02.2021 01:00



Mathematics, 03.02.2021 01:00


Chemistry, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00