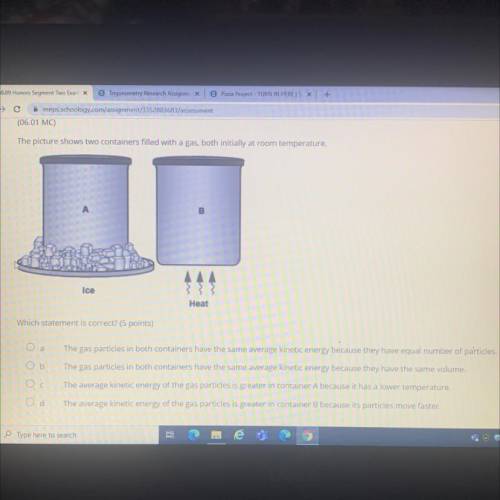
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice...

Chemistry, 15.12.2020 22:20 kendramiller3965
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice
Heat
Which statement is correct? (5 points)
О а
The gas particles in both containers have the same average kinetic energy because they have equal number of particles.
Ob
The gas particles in both containers have the same average kinetic energy because they have the same volume.
Ос
The average kinetic energy of the gas particles is greater in container A because it has a lower temperature.
The average kinetic energy of the gas particles is greater in container B because its particles move faster.
Od

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Questions

History, 28.06.2019 21:00


Mathematics, 28.06.2019 21:00




History, 28.06.2019 21:00

English, 28.06.2019 21:00





Mathematics, 28.06.2019 21:00


Mathematics, 28.06.2019 21:00

English, 28.06.2019 21:00

Computers and Technology, 28.06.2019 21:00

English, 28.06.2019 21:00


Computers and Technology, 28.06.2019 21:00



