
Chemistry, 15.12.2020 21:50 giulianna41
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxygen
according to the balanced reaction below. Your car uses the energy produced by this reaction
at a rate of 115 kJ per second while traveling at a speed of 65 miles per hour. Calculate the
distance (in miles) the car can travel using this amount of octane.
2 C8H18(g) + 25 O2(g) > 16 CO2(g) + 18 H2O(g) ΔHrxn = -10,900 kJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxy...
Questions

Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57




History, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57


Health, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57


Arts, 12.06.2020 22:57


Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57



Mathematics, 12.06.2020 22:57

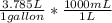 = 7380.75 mL
= 7380.75 mL

