

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen...
Questions

Mathematics, 05.10.2019 13:00

Mathematics, 05.10.2019 13:00



Biology, 05.10.2019 13:00


Biology, 05.10.2019 13:00


Mathematics, 05.10.2019 13:00

English, 05.10.2019 13:00

Social Studies, 05.10.2019 13:00

History, 05.10.2019 13:00

Computers and Technology, 05.10.2019 13:00


Mathematics, 05.10.2019 13:00

Biology, 05.10.2019 13:00

Mathematics, 05.10.2019 13:00



Biology, 05.10.2019 13:00


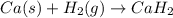
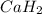 , it is -2.
, it is -2.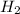 is zero and oxidation number of hydrogen in
is zero and oxidation number of hydrogen in 

