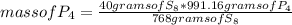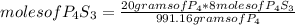Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
8P4 + 3S8 -> 8P4S3
The molar masses of the substances are as follows: P4 = 123.895g/mol, S8 = 25...
Questions

Mathematics, 02.03.2020 20:05

Mathematics, 02.03.2020 20:05









Chemistry, 02.03.2020 20:09






German, 02.03.2020 20:10

Spanish, 02.03.2020 20:10