
Chemistry, 15.12.2020 09:00 bcarri4073
QuesLIVII 2 PUNILS)
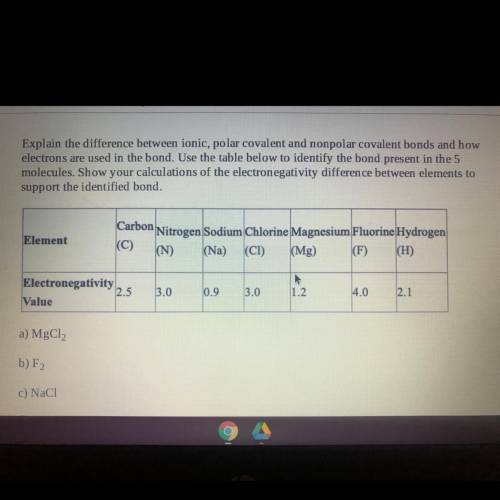
Explain the difference between ionic, polar covalent and nonpolar covalent bonds and how
electrons are used in the bond. Use the table below to identify the bond present in the 5
molecules. Show your calculations of the electronegativity difference between elements to
support the identified bond.
Element
Carbon
Nitrogen Sodium Chlorine Magnesium Fluorine Hydrogen
(N)
(Mg) (F) (H)
(Na)
(CI)
Electronegativity
2.5
Value
3.0
0.9
3.0
1.2
4.0
2.1
a) MgCl2
b) F2
c) NaCl
Sign Out

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

You know the right answer?
QuesLIVII 2 PUNILS)
Explain the difference between ionic, polar covalent and nonpolar covalent bond...
Questions








Business, 23.07.2019 03:50


Social Studies, 23.07.2019 03:50




Chemistry, 23.07.2019 03:50


Mathematics, 23.07.2019 03:50






