
Chemistry, 14.12.2020 20:30 clietmaster112
Gas law A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new pressure? The temperature in the number of moles stay the same.
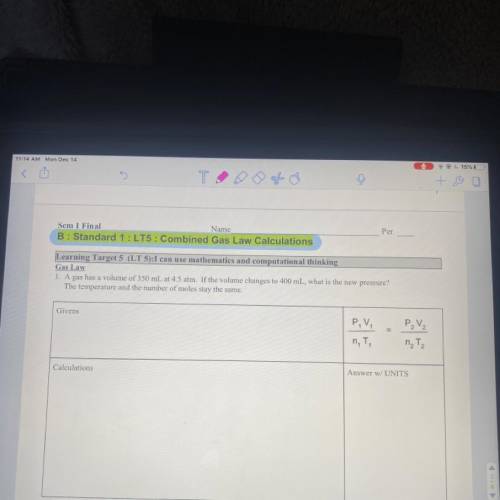

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
Gas law
A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new p...
Questions

Mathematics, 11.09.2021 04:10


Mathematics, 11.09.2021 04:10


Mathematics, 11.09.2021 04:10

History, 11.09.2021 04:10


Mathematics, 11.09.2021 04:10

Chemistry, 11.09.2021 04:10


Mathematics, 11.09.2021 04:10

Mathematics, 11.09.2021 04:10



Mathematics, 11.09.2021 04:20




Social Studies, 11.09.2021 04:20

Mathematics, 11.09.2021 04:20



