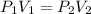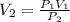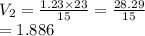Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1



Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
A gas starts at a volume of 23 L and a pressure of 1.23 atm. What is the new pressure if you
change...
Questions

Social Studies, 12.05.2021 17:20



Mathematics, 12.05.2021 17:20





History, 12.05.2021 17:20


Mathematics, 12.05.2021 17:20

Biology, 12.05.2021 17:20

Mathematics, 12.05.2021 17:20

History, 12.05.2021 17:20