
Chemistry, 14.12.2020 17:10 bjpvrpow74wq
You need 150g of pure lithium for an experiment you're doing. You have 675g of lithium oxide (Li2O). Can you extract all the lithium you need from the amount of compound you have? Show your reasoning.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
You need 150g of pure lithium for an experiment you're doing. You have 675g of lithium oxide (Li2O)....
Questions

Mathematics, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00

Chemistry, 10.11.2020 20:00

History, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00

Chemistry, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00

Arts, 10.11.2020 20:00

Mathematics, 10.11.2020 20:00


Chemistry, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00


Health, 10.11.2020 20:00

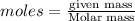
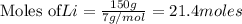
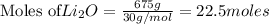
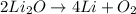
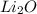 produce 4 moles of
produce 4 moles of 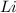
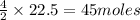 of Li
of Li

