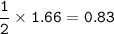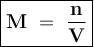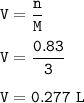Chemistry, 14.12.2020 14:00 rhaquan66766
Given the unbalanced equation below, answer the following: Calculate the number of liters of 3.00 M lead (II) iodide solution produced when 1.66 mol Kl react? Pb(NO3)2 + 2KI → 2KNO3 + PbI2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
Given the unbalanced equation below, answer the following: Calculate the number of liters of 3.00 M...
Questions


Social Studies, 11.12.2019 20:31

Mathematics, 11.12.2019 20:31

Biology, 11.12.2019 20:31




Mathematics, 11.12.2019 20:31




History, 11.12.2019 20:31


Biology, 11.12.2019 20:31

Mathematics, 11.12.2019 20:31



Mathematics, 11.12.2019 20:31


Mathematics, 11.12.2019 20:31