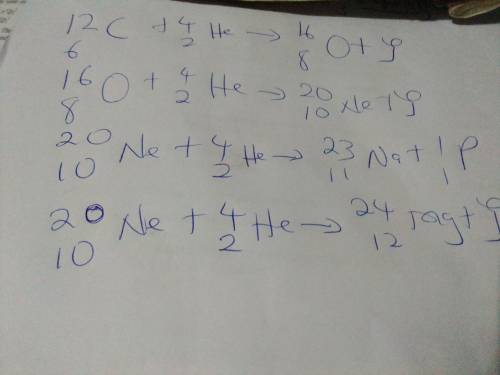Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
REG: ED 101/6/12062
Course Code:
.
1. Using appropriate chemical equations, explain stell...
.
1. Using appropriate chemical equations, explain stell...
Questions

Mathematics, 08.11.2019 08:31



Social Studies, 08.11.2019 08:31

Mathematics, 08.11.2019 08:31



Mathematics, 08.11.2019 08:31

History, 08.11.2019 08:31




Mathematics, 08.11.2019 08:31

Mathematics, 08.11.2019 08:31


History, 08.11.2019 08:31




Mathematics, 08.11.2019 08:31