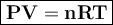Chemistry, 14.12.2020 03:20 335716hdjsngrhrs
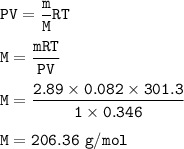
Calculate the molar mass of a 2.89 g gas at 346 ml, a temperature of 28.3 degrees Celsius, and a pressure of 760 mmHg.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Calculate the molar mass of a 2.89 g gas at 346 ml, a temperature of 28.3 degrees Celsius, and a pre...
Questions



Mathematics, 12.01.2020 00:31

English, 12.01.2020 00:31


Mathematics, 12.01.2020 00:31


Mathematics, 12.01.2020 00:31





Mathematics, 12.01.2020 00:31

Biology, 12.01.2020 00:31

Mathematics, 12.01.2020 00:31

History, 12.01.2020 00:31

Mathematics, 12.01.2020 00:31

English, 12.01.2020 00:31

Mathematics, 12.01.2020 00:31

Mathematics, 12.01.2020 00:31