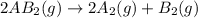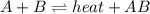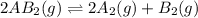1. chemical equilibrium is established when the number of reactants equals the number of products.. - true. - false. 2. according to le chatelier's principle, by increasing the temperature of the system shown below, the equilibrium will shift to the right (towards . a + b heat + ab. - true. - false. 3. for which of the following reactions will an increase in pressure not effect the position of equilibrium? . . a. 2a2 (g) + b2 (g) 2a2b (g). b. 2ab (g) a2 (g) + b2 (g). c. 2a (g) + f2 (g) 2af (g). d. 2b (s) + 2ha (aq) 2ba (aq) + h2 (g).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
1. chemical equilibrium is established when the number of reactants equals the number of products.....
Questions



Mathematics, 24.11.2020 07:40

Advanced Placement (AP), 24.11.2020 07:40

Social Studies, 24.11.2020 07:40


Mathematics, 24.11.2020 07:40




History, 24.11.2020 07:40

History, 24.11.2020 07:40



Mathematics, 24.11.2020 07:40



Mathematics, 24.11.2020 07:40

Mathematics, 24.11.2020 07:40

English, 24.11.2020 07:40