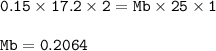Chemistry, 13.12.2020 23:20 bekahmc1p6k6vj
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
The student carried out a titration experiment to find the volume of 0.150 mol/dmº
H2SO4 needed to neutralise the KOH.
The student found that, on average, 17.20 cm of the H2SO4 solution was
required for neutralisation.
Calculate the concentration of the KOH solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1


Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
Th...
Th...
Questions

Physics, 21.08.2019 18:10

Computers and Technology, 21.08.2019 18:10


Computers and Technology, 21.08.2019 18:10




Computers and Technology, 21.08.2019 18:10


Mathematics, 21.08.2019 18:10




History, 21.08.2019 18:10




Biology, 21.08.2019 18:10