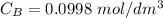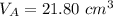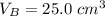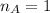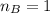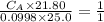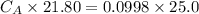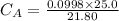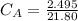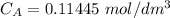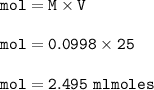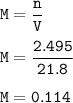Student carries out a titration to determine the concentration of a solution of
nitric acid. She titrates the solution of nitric acid against a standard solution
of sodium hydroxide with a known concentration of 0.0998 mol/dm². She
finds that 21.80 cm of the nitric acid solution is needed to exactly neutralise
25.0 cm of the sodium hydroxide solution.
Calculate the concentration of the nitric acid solution. Give your answer to
three significant figures.
The equation for the neutralisation reaction is
HNO3 + NaOH → NaNO3 + H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

You know the right answer?
Student carries out a titration to determine the concentration of a solution of
nitric acid. She ti...
Questions

Chemistry, 03.04.2020 01:28

Mathematics, 03.04.2020 01:28

Biology, 03.04.2020 01:28


Mathematics, 03.04.2020 01:28

History, 03.04.2020 01:28


Mathematics, 03.04.2020 01:28

English, 03.04.2020 01:28

Social Studies, 03.04.2020 01:28

Mathematics, 03.04.2020 01:28



Chemistry, 03.04.2020 01:28




Mathematics, 03.04.2020 01:29

Health, 03.04.2020 01:29

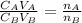
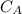 is the concentration of acid
is the concentration of acid
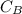 is the concentration of base
is the concentration of base
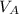 is the volume of acid
is the volume of acid
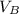 is the volume of base
is the volume of base
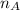 is the mole ratio of acid
is the mole ratio of acid
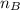 is the mole ratio of base
is the mole ratio of base