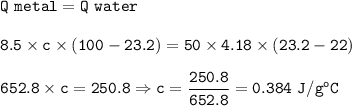An unknown metal with a mass of 8.5 g was heated in boiling water to a temperature of 100°C. The metal was immediately transferred to an insulated cup containing 50.0 g of water at 22°C. At equilibrium (when the temperature became constant) the temperature of the system was 23.2°C. Calculate the specific heat of the metal and determine its identity. Explain how you arrived at your conclusion. You must show your work to receive credit for your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
An unknown metal with a mass of 8.5 g was heated in boiling water to a temperature of 100°C. The met...
Questions


English, 16.10.2020 18:01




English, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01





Computers and Technology, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01